The demand for environmental goods such as safe food, clean air and safe drinking water is often low in developing countries. It is clear that one of the major causes of low demand is poverty, which reflects choices made by individuals in their own best interests. Poor quality of drinking water is a major health hazard in developing countries and most of the fatal diseases are associated with it especially among the children.
Sri Lankan Context : Ground Water Hardness
Sri Lanka’s continued efforts to improve its social development indicators have placed the country ahead of most other South Asian countries. Provision of drinking water supply is a government priority and targets have been set periodically with regards to population access to safe drinking water. On a nation- wide basis, piped water systems and protected wells deliver safe water to almost 90% of the urban population and 60% of the rural population. Piped water is supplied to 31% of the population at present which is over 6 million people. Tube wells provide water to a population of more than 2 million (10% percent). In addition, 27 percent of the population living in rural areas has been provided with safe drinking water through protected dug wells. Accordingly, 90 percent of the urban population and 60 percent of the rural population are provided with safe drinking water facilities.
Challenges in Ground Water Quality
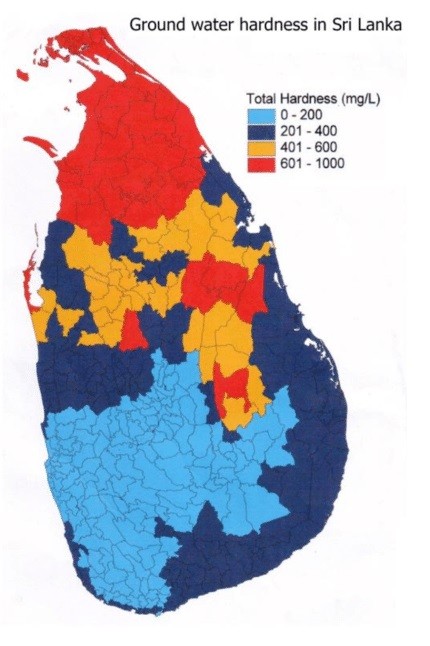
Above map shows the hardness distribution of groundwater in Sri Lanka. Over 60% of groundwater in Sri Lanka exceeds 200 PPM, the hardness level literally not safe for drinking. Hence groundwater hardness level severely challenges the goals set by governing authorities towards safe water for the people.
Water Purification for Hard Water
Hardness is explained by the occurrence of Calcium and Magnesium Compounds; carbonates and bicarbonates in water. Water softening through Ion exchange resins is the most efficient method available globally. In this process Ca2+ and Mg2+ exchange ions to Na+ or H+.
Cation exchange resin is mainly designed for the industrial water softening and demineralization applied in power plant etc., also can be used in house-hold water conditioning equipment. The required capacity of the softener system is purely depending on Hardness level of water and daily capacity (water consumption).